Strategic Partnerships
Making medicines together.
We strategically partner with groups that have novel science and innovative technology to develop antibody-based medicines.
- Regeneron
- Lilly
- Moderna
- Novartis
- Abdera
- Denali
- Incyte
- IGM
- Teva
- Versant
- Regeneron
- Lilly
- Moderna
- Novartis
- Abdera
- Denali
- Incyte
- IGM
- Teva
- Versant
- Regeneron
- Lilly
- Moderna
- Novartis
- Abdera
- Denali
- Incyte
- IGM
- Teva
- Versant
- Regeneron
- Lilly
- Moderna
- Novartis
- Abdera
- Denali
- Incyte
- IGM
- Teva
- Versant
Collaborating to develop first-in-class and best-in-class antibody therapeutics.
Our engine integrates expert teams, technology, and facilities with the data science and automation needed to advance antibody-based medicines from target to clinic with greater precision and speed.
We have developed specific technology platforms to unlock high-value drug classes, targets, and modalities, including T-cell engagers and transmembrane proteins.
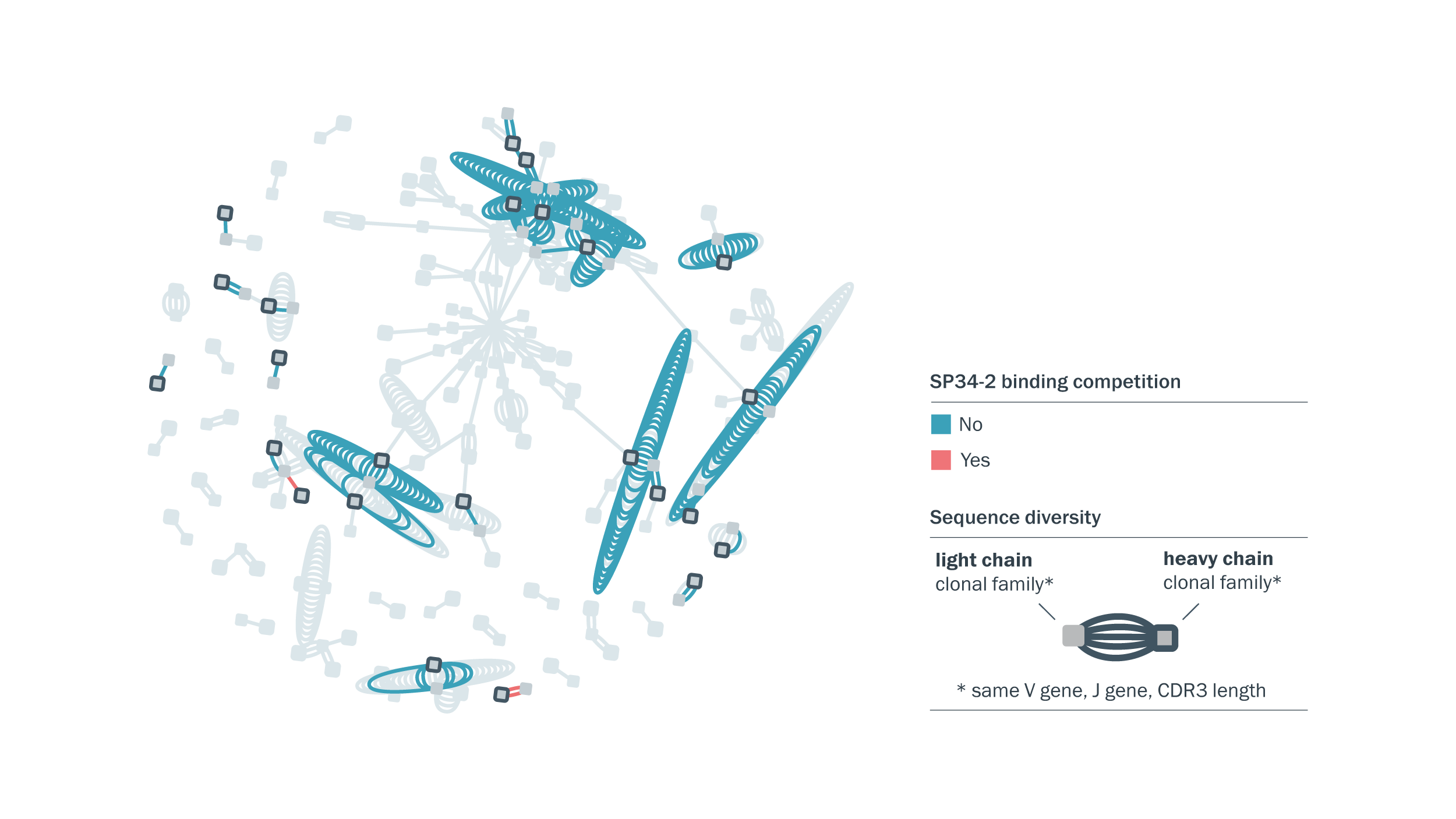
Our fully human CD3-binding antibodies are distinct from commonly used molecules. Antibody sequence diversity was visualized using Celium™ and is colored based on binding competition with SP34-2, a commonly used CD3-binding antibody for T-cell engager development.
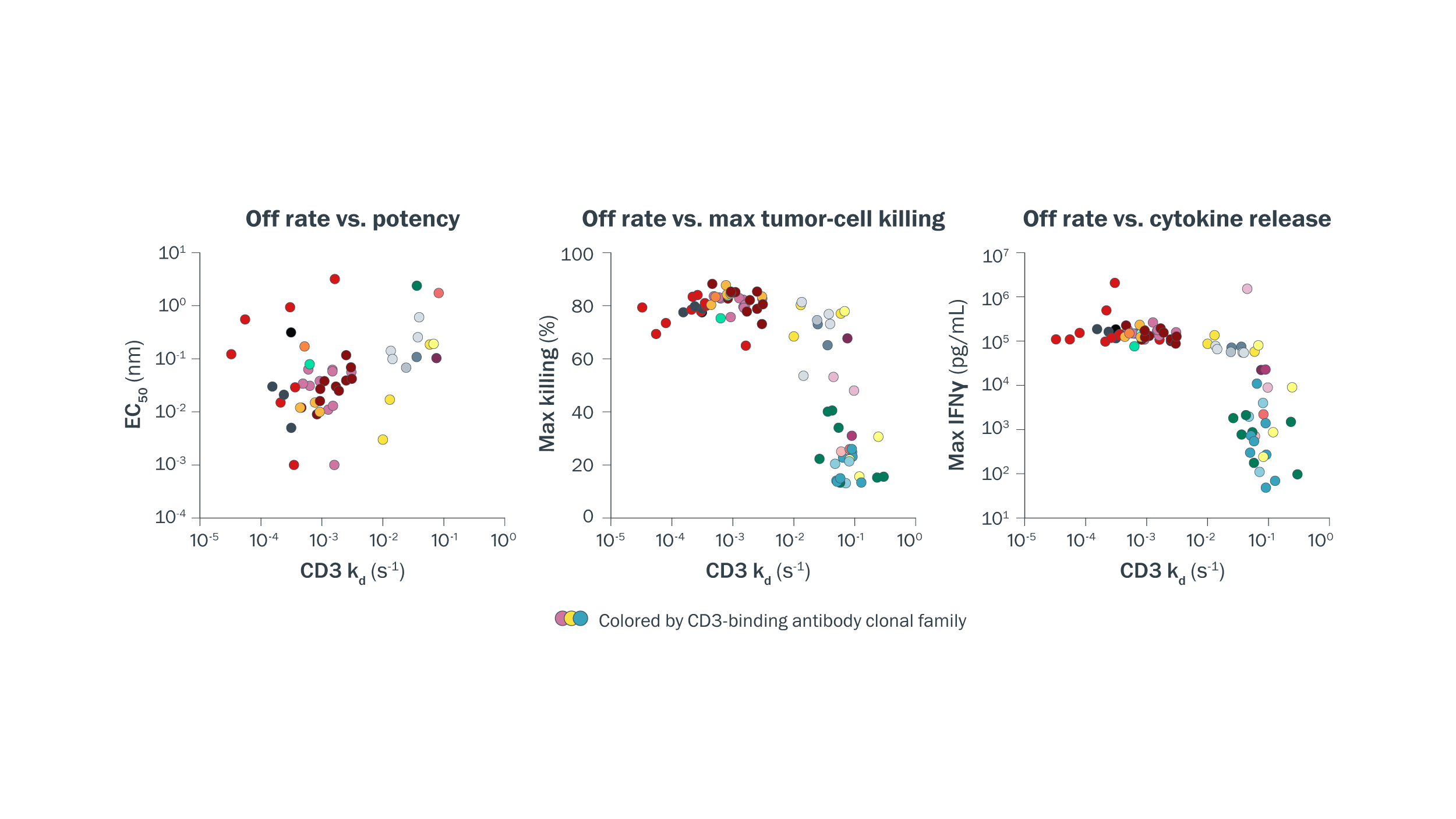
T-cell engager function is determined by multiple factors, including—but not limited to—CD3-binding kinetics. Binding kinetics for the human CD3 εδ subunit were compared to T–cell engager potency, tumor-cell killing, and cytokine release. Off rate was found to be a significant contributor to cytokine release and tumor-cell killing, but was not sufficient to fully describe these properties, particularly at faster off rates.
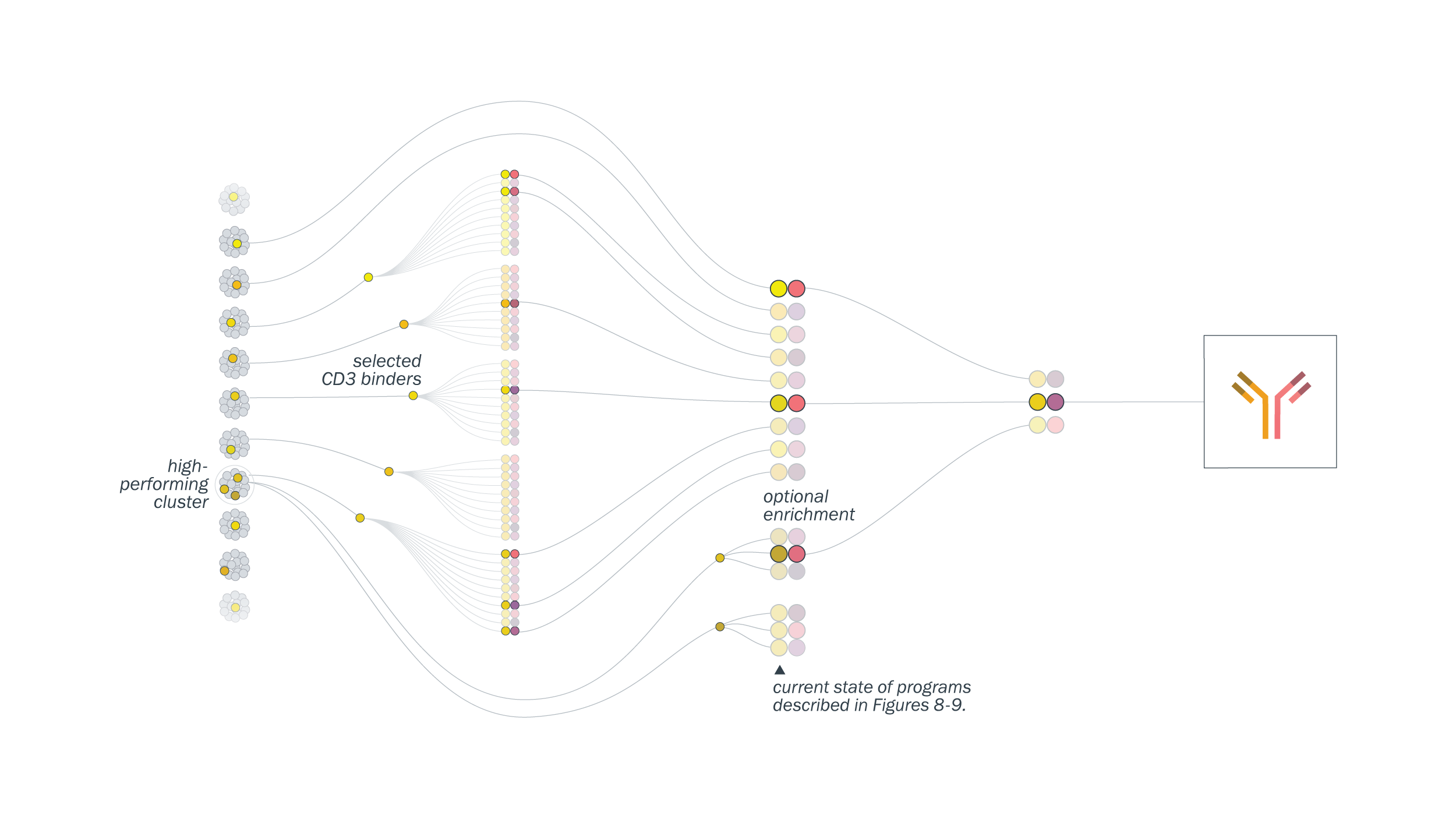
We take a function-first approach for T-cell engager development. We performed a functional clustering analysis that allows us to select functionally diverse CD3-binding antibodies for each tumor target. We engineer and analyze hundreds of bispecifics in high-throughput and down-select for those with desired properties.
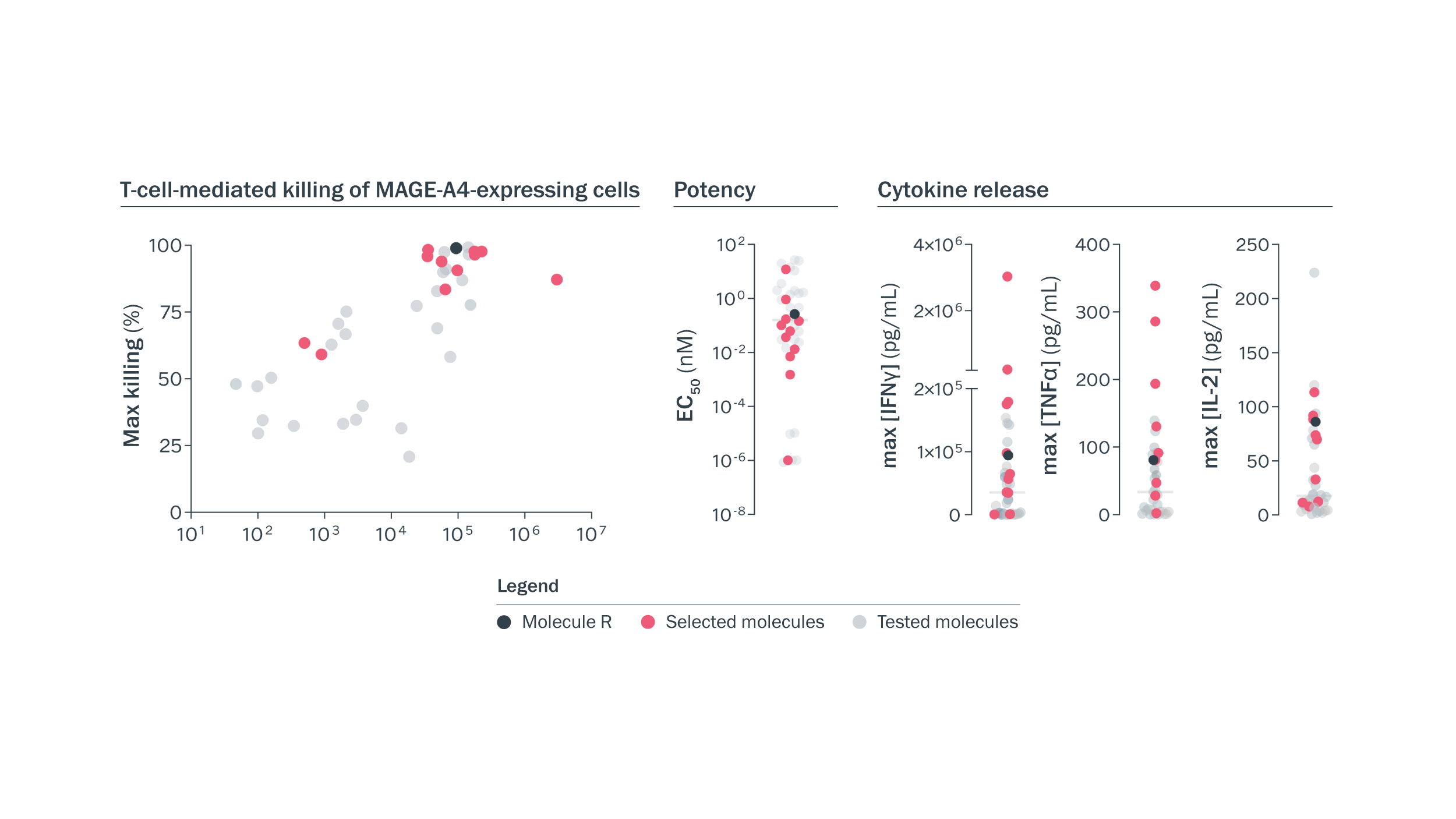
Functional MAGE-A4 x CD3 T-cell engagers were identified using high-throughput assessments. T-cell-mediated killing of wild-type and MAGE-A4 knockout cells was measured in a high-throughput assay. We assessed more than 200 1×1 MAGE-A4 x CD3 molecules alongside a 2×1 benchmark molecule, and 12 with desired functional activity were selected for further assessment.
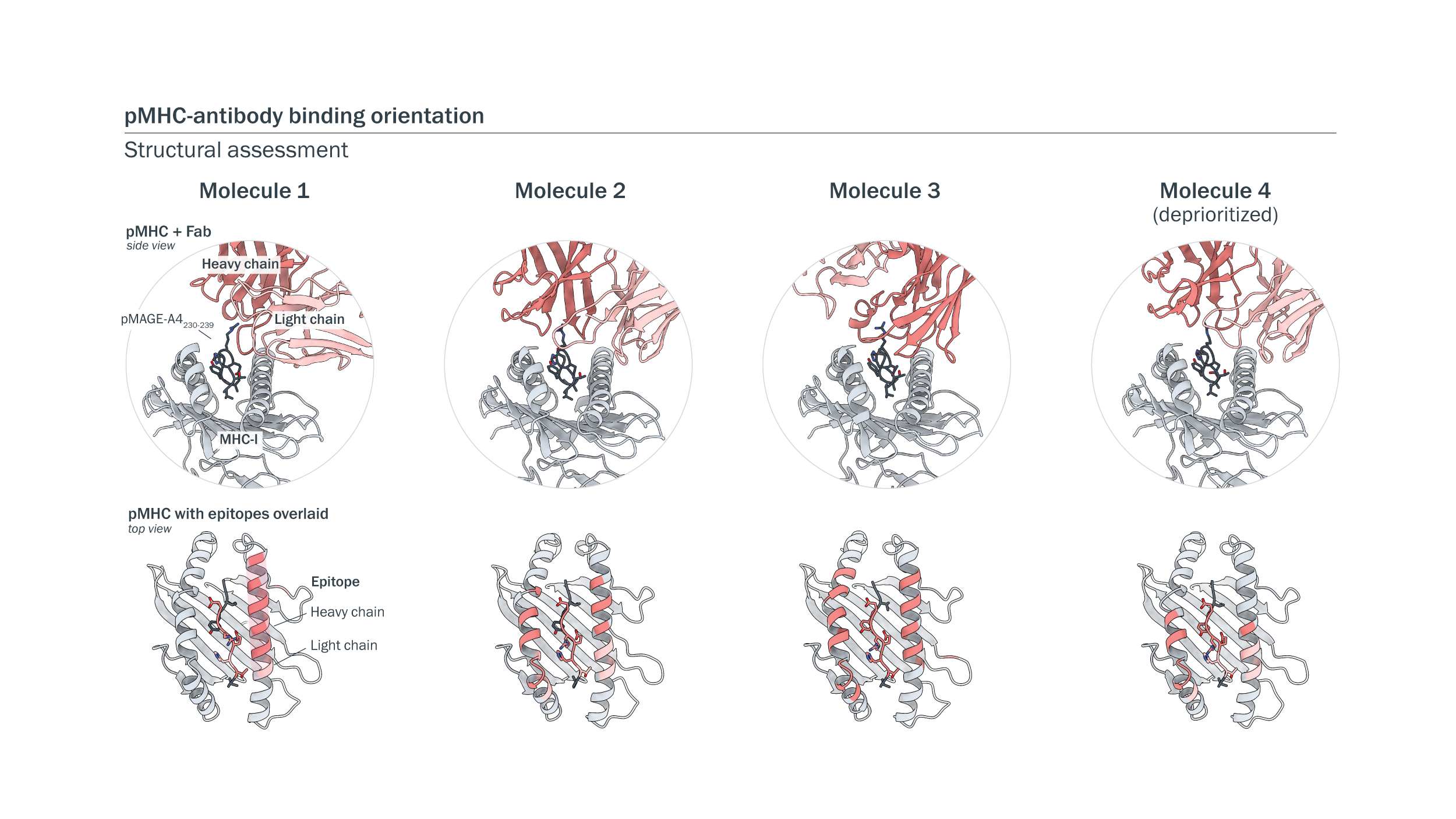
Structural analyses of pMHC-binding arms revealed diverse binding orientations. We assessed the structures of antibody Fabs bound to pMAGE-A4 displayed on MHC-I using X-ray crystallography and cry-electron microscopy at 2.7 to 3.3 Å. Antibodies showed peptide-centric binding to MAGE-A4-pMHC at diverse angles and orientations.
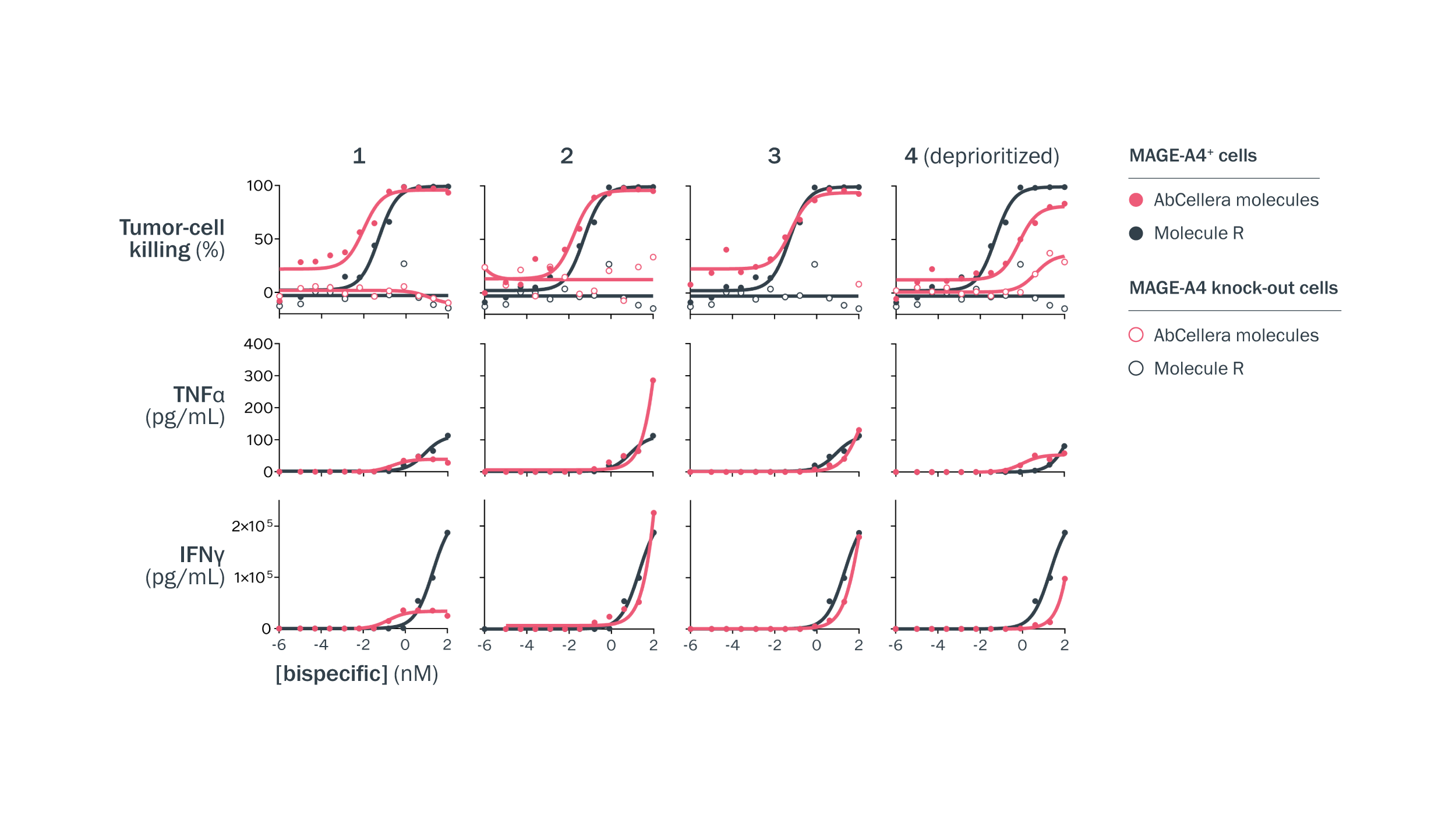
T-cell engagers with highly specific targeting of pMHC-expressing cells were selected for further assessment. T-cell-mediated killing of MAGE-A4-expressing A375 cells and A375 MAGE-A4 knockout cells was measured in a high-throughput assay. Molecule 4 is an example of a TCE that was deprioritized due to killing of MAGE-A4 knockout cells at higher antibody concentrations.
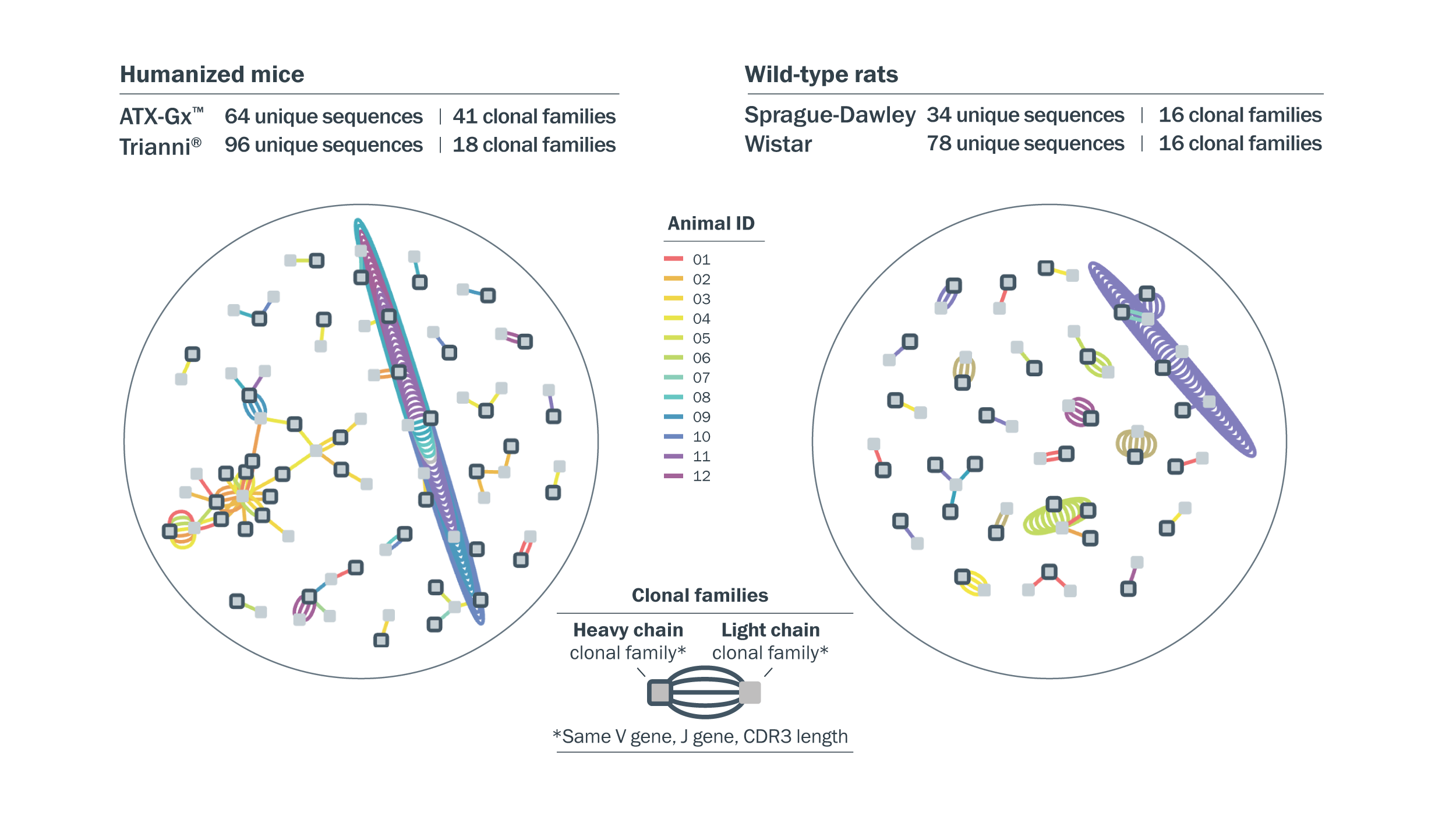
272 diverse CCR8-specific antibodies were identified from immunization and deep single-cell screening. Antibody sequence diversity was visualized using Celium™, and 272 CCR8-binding antibodies were selected for high-throughput expression based on clonal diversity.
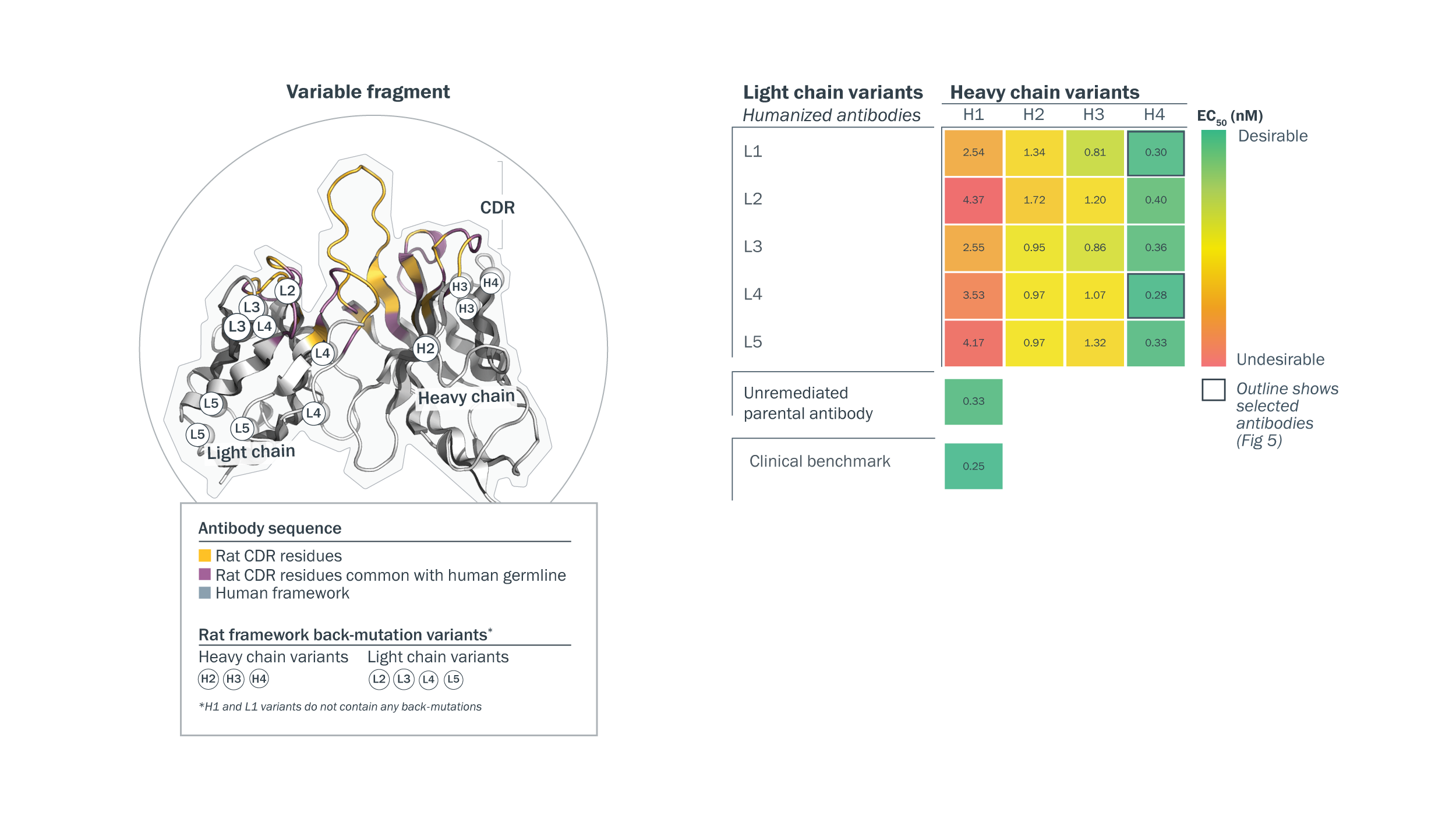
Structure-guided humanization was used to identify candidates with optimal profiles for predicted immunogenicity and function. Homology modeling guided the assessment of residues targeted for humanized back-mutations on the light (L1-5) and heavy chains (H1-4). The impact of heavy/light chain back-mutations on function was assessed in a rational, combinatorial approach and measured in an ADCC reporter assay.
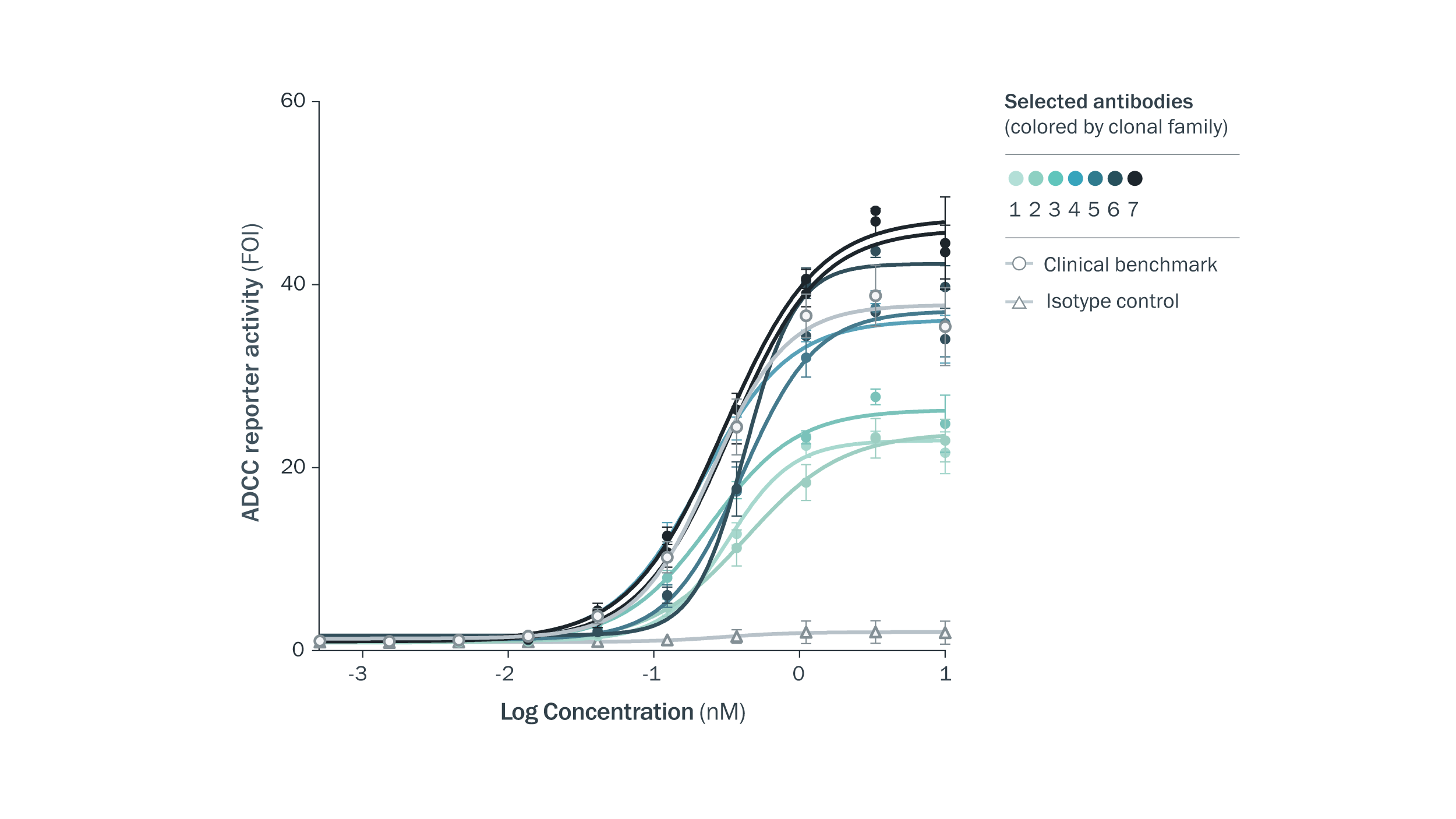
Strategic selection of diverse, developable antibodies with a range of potencies. Selected antibodies were similar to a clinical benchmark molecule in an ADCC reporter assay.
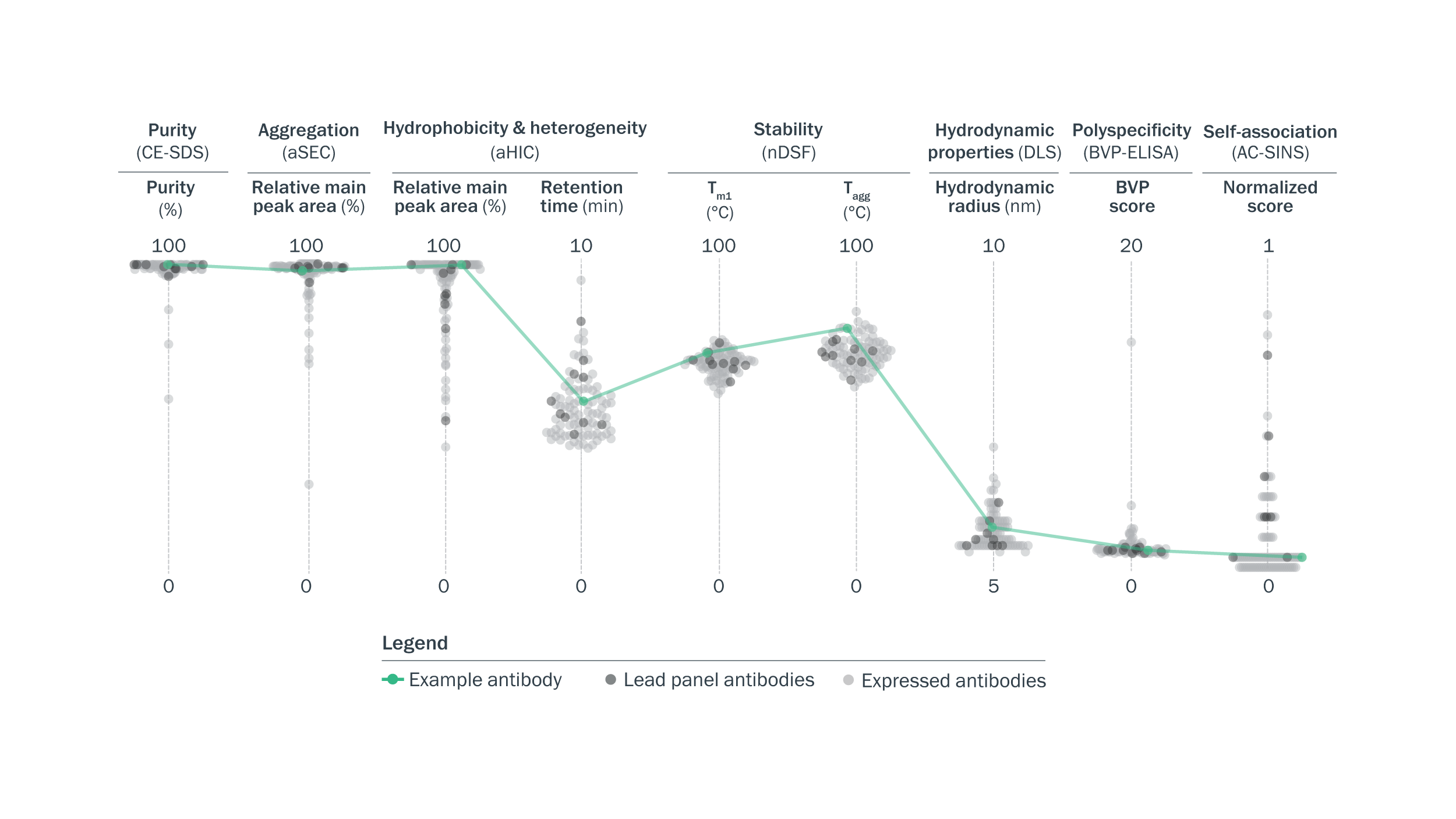
Lead panel antibodies showed favorable developability profiles. Selected lead panel antibodies showed high purity and stability, low aggregation, relative surface hydrophobicity, polyspecificity, self-association, and heterogeneity, and typical hydrodynamic properties.
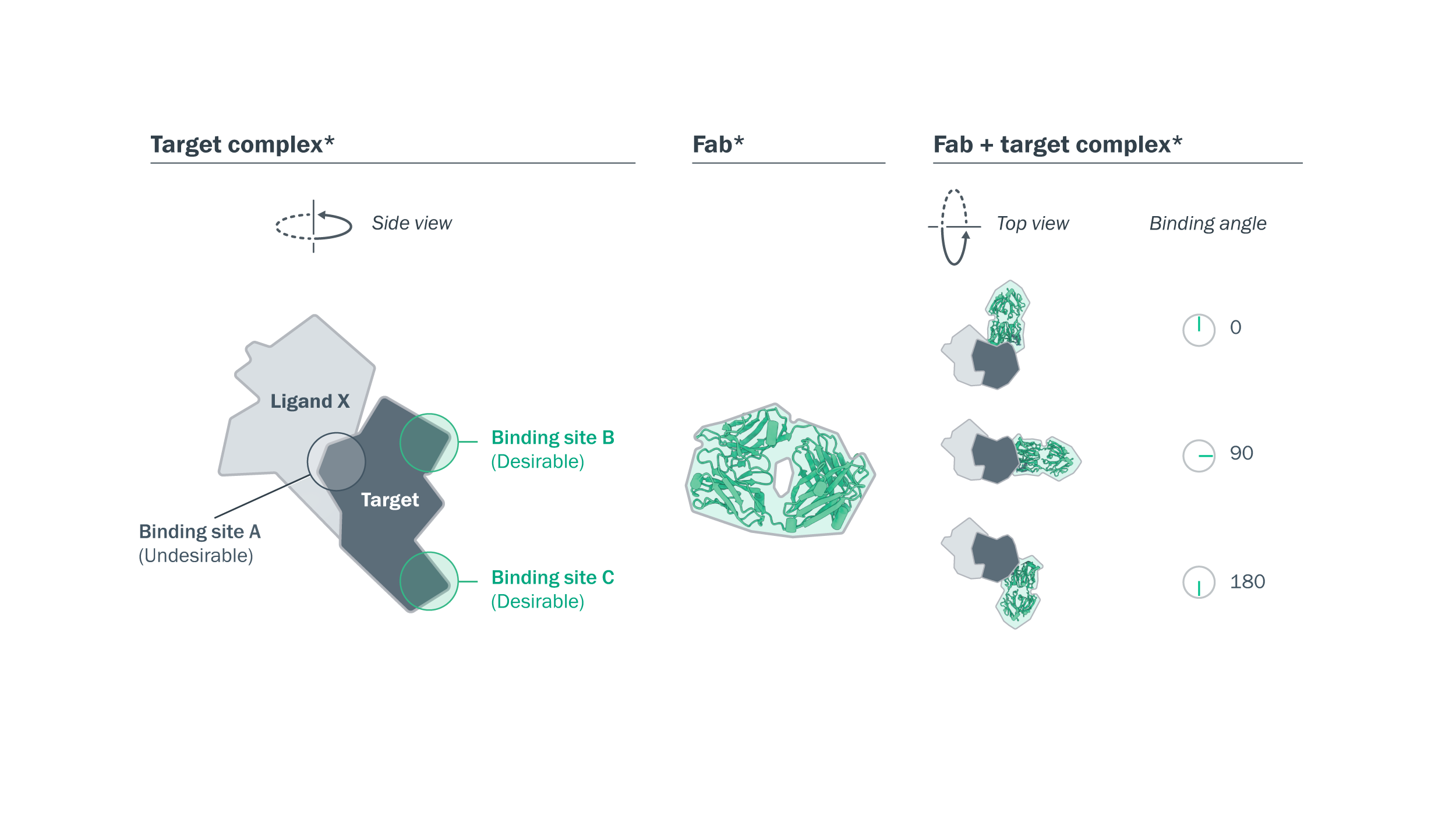
3D modeling revealed desirable structural binding profiles in 11/12 lead panel antibodies, and diversity in binding complex. Structural models were generated using SEC and negative stain electron microscopy. The side view shows an illustrative structure of the Fab and target. The top view shows the range of Fab binding angle of approach.
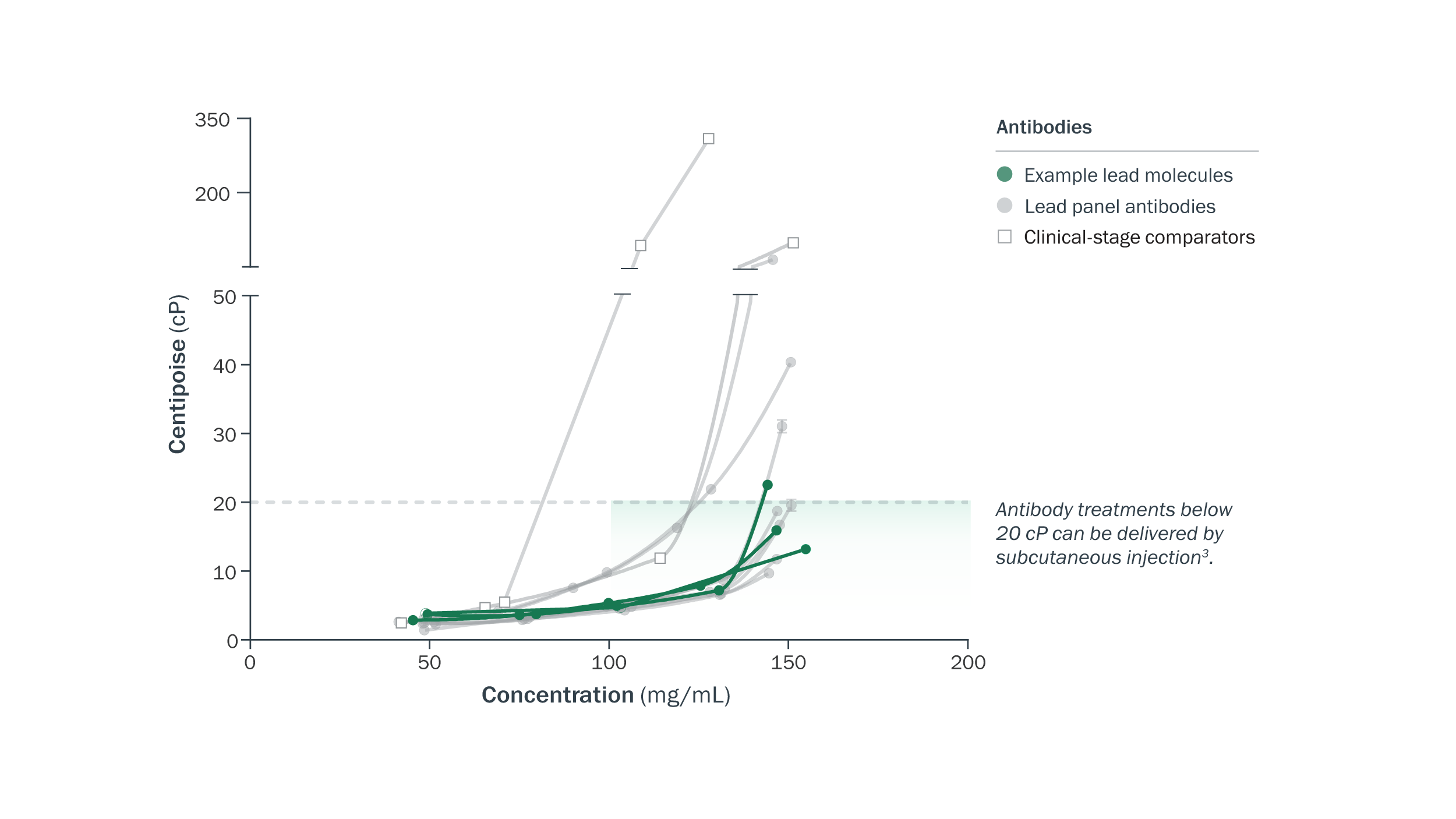
Potential for subcutaneous delivery was confirmed for multiple antibodies, with low viscosities at high concentrations. Dynamic viscosity was assessed at concentrations up to 150 mg/mL and compared to a panel of clinical-stage comparator antibodies.
Making a difference for patients.
Since 2015, we have worked with more than 40 partners on 100+ antibody drug programs, with 10 candidates reaching the clinic to date.
Antibody Drug
Programs Started
Partners
Candidates Reached
the Clinic
Patients Treated Worldwide
Engaging in strategic partnerships.
We look to create value with partners that bring insights into novel biology or technology, creating new opportunities in therapeutic areas of shared interest.
Let's Talk
Working together.
We partner with innovative biotechs and leading pharmaceutical companies to tackle the toughest problems in drug development.
“AbCellera’s technology enables us to extend our competitive advantage in target discovery and validation with best-in-class antibodies to rapidly advance our programs towards the clinic. We are excited to expand our relationship with AbCellera and to translate many more genetically validated targets into potential new antibody-based therapies together.”
Omri Gottesman, MD / CEO and President of Empirico
“We are seeing a wave of innovation in the antibody space that is allowing us to add novel functionalities to these molecules. Our partnership with AbCellera will further enable our portfolio companies to pursue these important biologic medicines.”
Markus Enzelberger, PhD / Partner at Versant Ventures
“We continue to be impressed with the speed of discovery, the quality, and the diversity of the antibodies AbCellera delivers. Through this agreement, we have secured expanded access to an industry-leading technology to accelerate the discovery of antibody-based therapies for patients with neurological diseases.”
Alexander Schuth / COO of Denali
“AbCellera’s clinically validated platform lets us start discovery in a virtualized model that aligns well with our capital efficient investment strategy. This partnership supports our approach to value creation by allowing us to focus on building companies that aim to deliver impactful medicines to patients.”
Steven Robinette, PhD / Venture Partner at Atlas Venture
Strategic partnerships.