
Our Focus
First-in-class and best-in-class antibody medicines.
We are advancing programs to address unmet medical need in autoimmunity, endocrine and metabolic conditions, and beyond.
We are leveraging our engine to generate a pipeline of programs with first-in-class and best-in-class potential. Our first two preclinical programs, ABCL575 and ABCL635, are currently in IND*-enabling studies.
In addition, we anticipate additional development candidates will enter IND-enabling studies in 2025.
*IND = Investigational New Drug application in the United States. Applications may be submitted to other jurisdictions, including Clinical Trial Applications (CTA) in Canada.
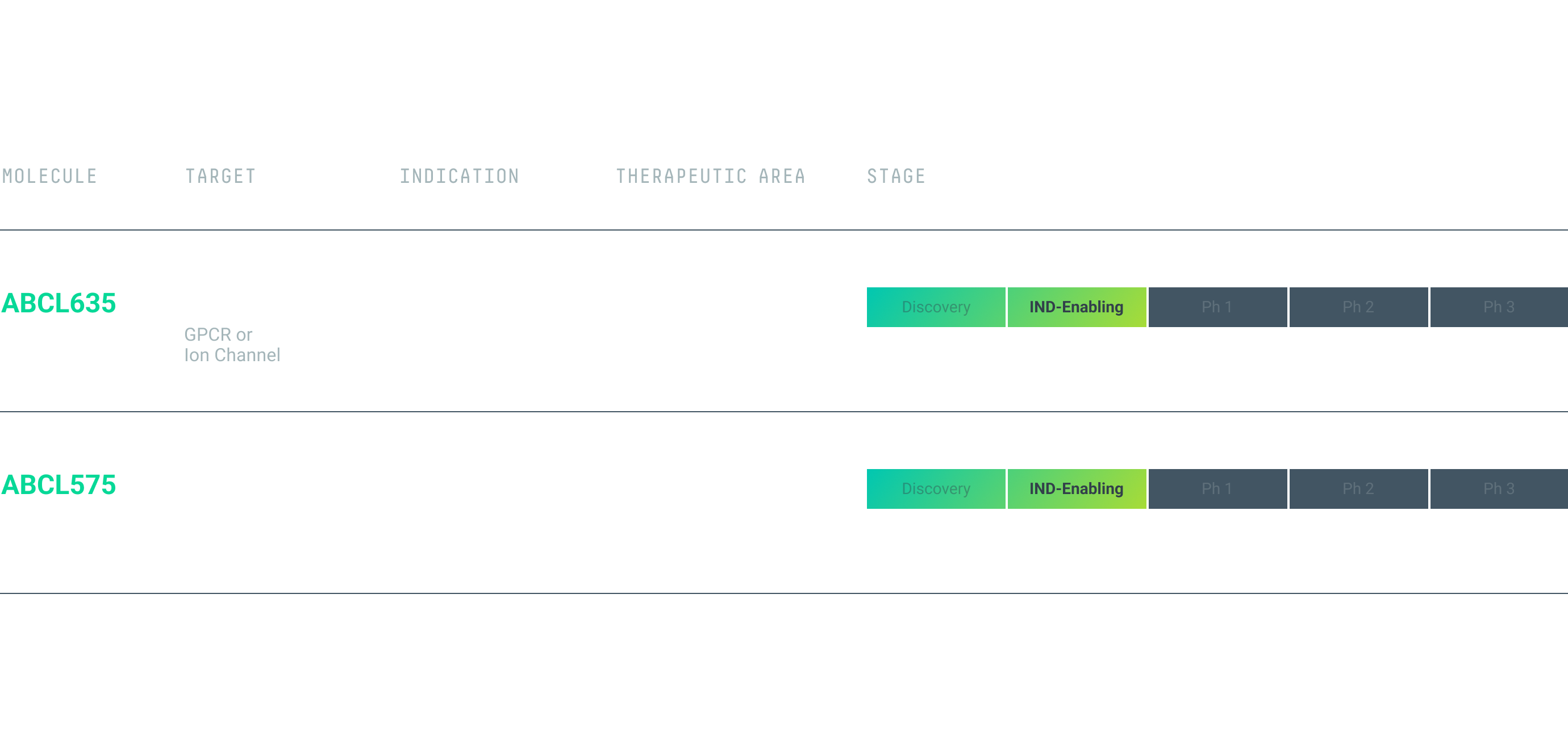
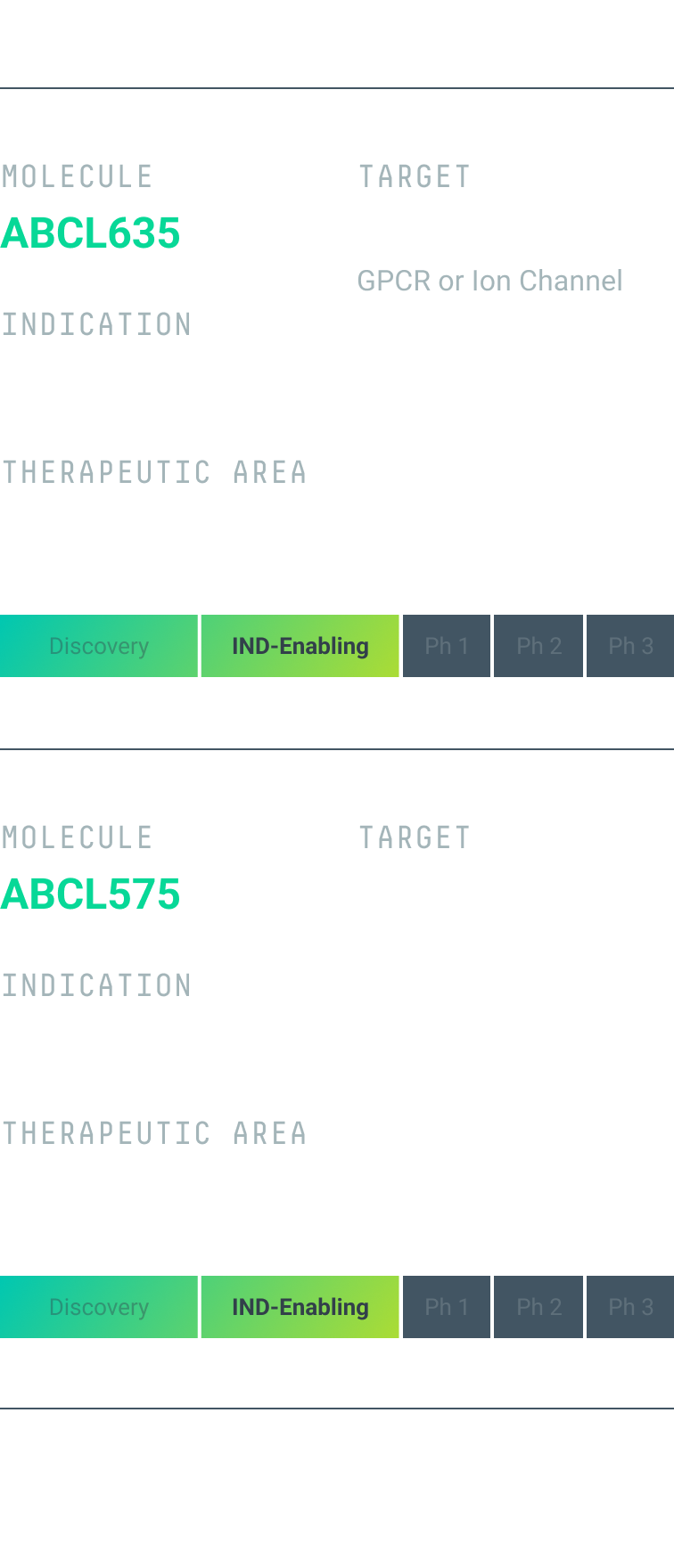
ABCL635
A potential first-in-class medicine for metabolic and endocrine conditions.
ABCL635 is an antibody drug candidate targeting an undisclosed multipass complex membrane protein with an indication in metabolic and endocrine conditions.
ABCL635 is the first AbCellera-led asset derived from our GPCR- and ion-channel platform. We anticipate clinical trial application submission in Q2 2025.

ABCL575
A potential best-in-class medicine for atopic dermatitis.
ABCL575 is a fully human, half-life extended monoclonal antibody targeting OX40L as a potential best-in-class treatment for T-cell-mediated autoimmune conditions, such as atopic dermatitis. Antibody-mediated blockade of OX40L is a clinically validated, non-T cell depleting mechanism to modulate inflammation. ABCL575 has been designed with potency, pharmacokinetics, and developability to support less frequent dosing. The program is in IND-enabling studies and we anticipate clinical trial application submission in Q2 2025.